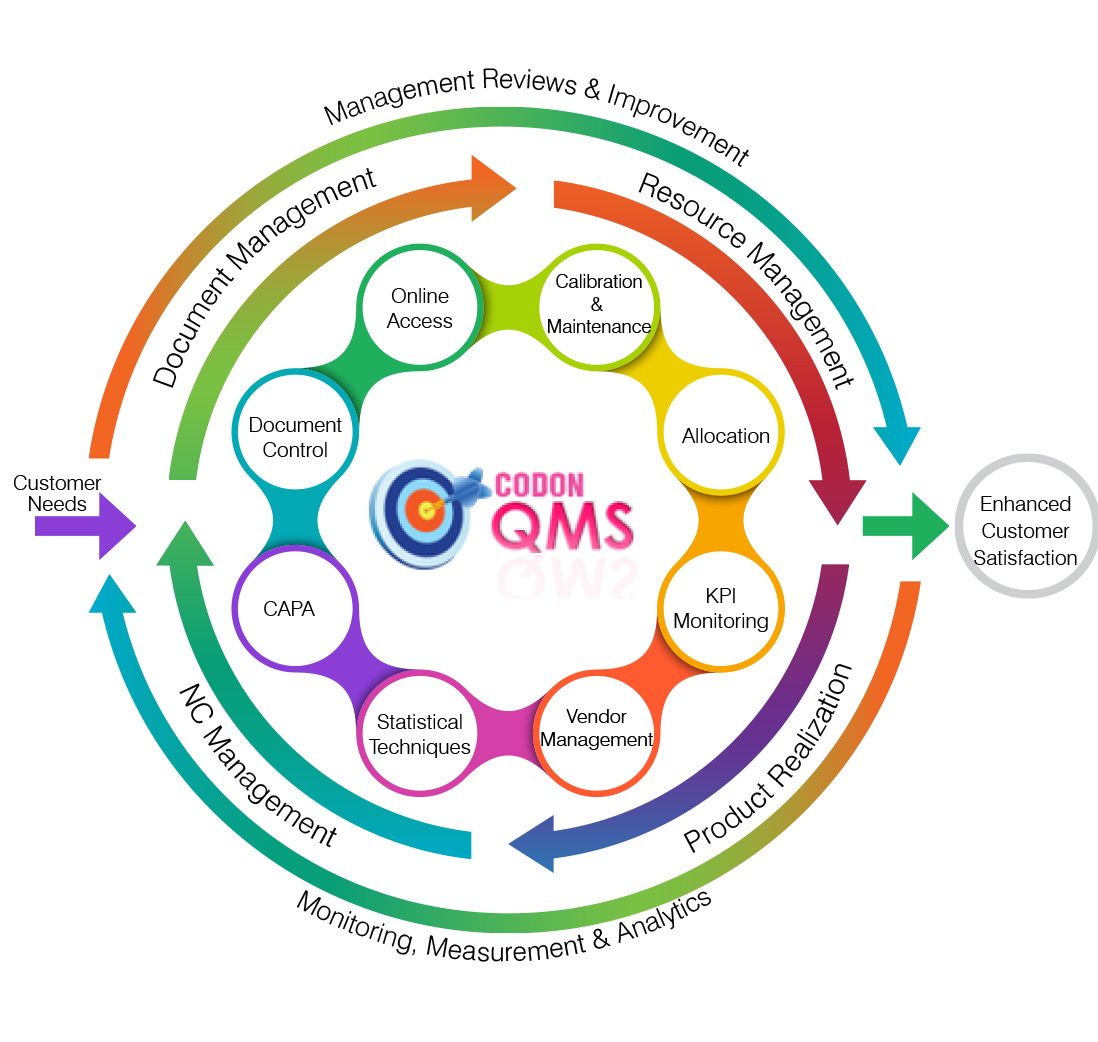

Document Management
(Clause 4.4 & 7.5)
Having necessary documented information is important for the effective
implementation and maintenance of QMS. CodonQMS provides the 'Documents' feature
that allows easy maintenance & control of documents. The documents can be uploaded
or directly made on-line and are approved through a workflow. Control of changes is managed
using version/revision number and revisions will be approved through a work flow system before being made available all.
An amendment record is maintained for Level 1 & Level 2 documents. Context of the organization (4.1), Expectations of the interested parties (4.2), Quality Policy (5.2) and Risks & Opportunities (6.1)
are posted separately and made available on the main Menu bar for effective communication and quick reference.
Documents like Quality Policy are made available on the main
Menu bar for effective communication within the organization.

Resource Management
(clause 7.1.3)
For every enterprise, resources are needed and must be used efficiently and effectively for optimal utilization and organizational growth. CodonQMS automates and assists the process of resource (equipment & transportation) allocation and provides resource transparency such as financial resources, inventory, human skills etc. It enables viewing of resources status/ stocks and to take decisions on allocation to newer projects for effective management at optimum cost. This feature also captures the preventive maintenance schedules and breakdown maintenance details for efficient resources management.

Calibration
(Clause 7.1.5)
Calibration of monitoring & measuring resources/devices is critical to ensuring fitness of the devices and correctness of data. CodonQMS has the provision to capture all calibration requirements, calibration status and to monitor & control their validity for use. Also captures the calibration lab details and their credentials like NABL certification etc

Operation/Design, production, Delivery (Clause 8.0)
While the 'Operations' are the doing activities, CodonQMS - Documents provide the necessary information for doing them right all the time. The Documented information is made available on-line for effective use. The CodonQMS can capture the results of these activities - ie design, production, release of products & services and post-delivery activities etc for effective management & control. This makes the processes robust and more reliable delivering the required results. CodonQMS is an effective Quality Management System includes a comprehensive approach to getting from the product concept to the finished product.It specifically involves in quality objectives like planning, design control and product control.

Analysis & Evaluation
(Clause 9.0)
Monitoring & Measurement and Performance Evaluation are very important to achieve
desired results. All critical data including Non-conformances/Incidents are captured using this application
and analysis reports/trend analysis are made available to the Management. While CodonQMS captures various information processed through this application and provide analysis, it also processes data that are fed into the system based on Management decision. This will help Leadership / Management to review & evaluate all key performances as defined by the organization, making them to ensure that these processes and systems are delivering required results and non-conformities are getting addressed on-time.

Internal Audit and Management
Review
(Clause 9.2 & 9.3)
Conducting Internal audits at planned intervals are important to verify the established quality management
system conformance to the international standard and its effective implementation. The information related to Internal audits planning is captured
on-line and audit observation reports are uploaded for record. The non-conformances are created on-line and that drives the NC closure on-time and
effective implementation and maintenance of QMS.
The module also have the Management Review feature where the MRM report can be uploaded and Action points are created on-line.
This enables the process owners to act and close the action points resulting in improvements and sustainable performance.
Non-conformity, Corrective Action, Improvement
(Clause10.0)
Performance Evaluation, Corrective Actions and Improvement are
highly critical for enhancing customer satisfaction and ensuring sustainable growth.
CodonQMS has a feature/process to capture all types of Non-conformances/Incidents and to arrive
at the corrective actions / improvements within a stipulated time. Provides a systematic approach
throughout the organization for effective root cause analysis and that enables arriving at accurate
corrective actions. Severity of the problem (Low/Medium/High) can be defined by the organization to bring focus to
critical issues. Provides daily / weekly reports on status & pending issues to ensure timely resolution. Drives accountability
ensuring the actions are taken within the specified duration. Tracks the team member's responses and gives alerts for timely
actions Customerwise complaints can be monitored enabling timely resolution of problems.
The Suggestions data that is part of 'Continual Improvement' is made available under Internal Audit module.
This enables driving the improvement culture across the organization